Compliance pressures in pharma have never been higher. The FDA issued 3,344 observations to pharma companies in 2018. The best time to comply with cGMP for quality isn’t after you’ve failed an inspection, it’s before you receive a warning.
One way to ensure that a 483 doesn’t show up in your mailbox is by understanding what the most common compliance issues in the pharmaceutical industry are so that you can focus on those areas.
Why GenBioCa?
Use GenBioCa enriched Hybrid compliance model that can deliver a toolkit for your business compliance.
First Time Right (FTR)
- Penalties for non-compliance have financial and non-tangible bearing.
- Loss of reputation as a result of observations by regulatory authorities.
Systematic Approach
- Burden on management for audits if a systematic approach is not followed. This would involve lot of man-hours being lost, especially of senior responsible personnel, in the preparatory activities for audits.
- Increased emphasis on quality documentation, reviews and approvals for various artefacts needed as documentary evidence for the inspections and audits, if a smooth mechanism is not in place.
- Investigations and preparation of reports for any observations, replies to queries from the regulator if the audit is unsatisfactory can lead to teams being occupied for considerable time
Our Spectrum of Work
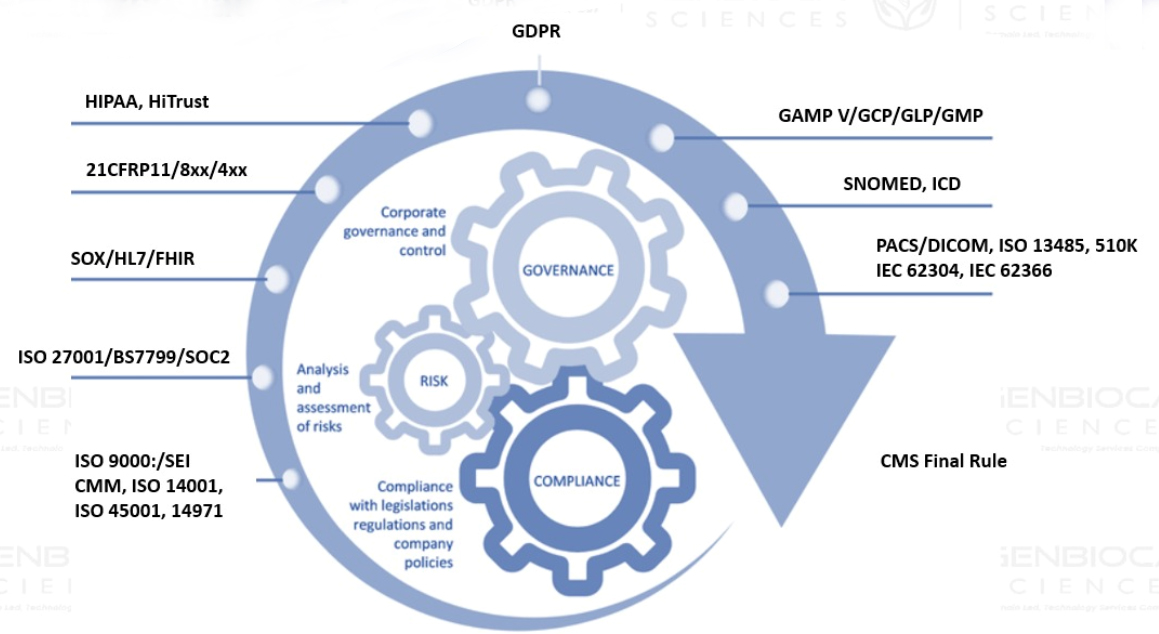
- GxP Compliance, Compliance to regulatory standards – GDPR, 21 CFR P11, SOC2, FHIR, HIPAA, 5010K, 13483
- Gap Analysis, CAPA, Mock Audits, Internal audits, Surveillance audits
- Training, Addressing Warning letters
- SOP Management (Creation, Revisions, Training, Software applications)
- Conducting Internal Audits (Facilities, Equipment and instruments, QC & QA functions, Manufacturing Processes, Maintenance activities (Preventive and Corrective)
- Quality Management Systems (Documentation, Training)
- Good Laboratory Practices (Laboratory Documentation, Waste Management)
- Good Manufacturing Practices (Documentation, Change Management, Corrective and Preventive Actions, Trend Monitoring, analysis, Quality by design, Quality Improvement Methodology)
- Management of Software applications in the organization (Surveys for effective usage, Documentation, Vendor Assessment Protocols, Guidance and training, CSV documentation)
- Filing NDA/ANDA, Submissions, e-CTD
- Safety and Hygiene Management in labs Laboratory Support (Resources and training)
For more information, please refer Regulatory Compliance Brochure